We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Clinical data
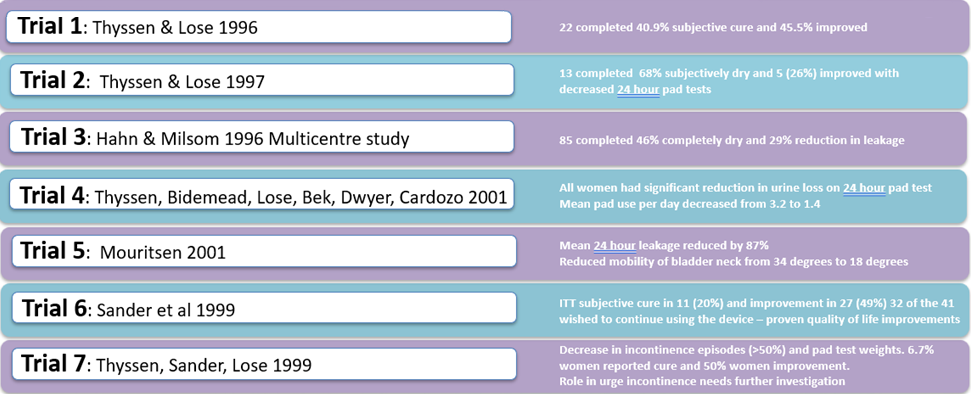
The success of Contrelle Activgard is clinically evidenced by seven clinical trials to date, all with statistical significance.


Data demonstrates high levels of efficacy, with long term (12 month) data showing 94% of women report an improvement in symptoms.
Clinical data reports significant reduction in pad usage/ volume of leakage, with significant improvement across objective scores and major improvement in quality of life.
Additionally, Scandinavian women have been using Contrelle® for over 10 years, with approaching 6 million devices used.
There have been no reports of any issues with Toxic Shock Syndrome.
Clinical data shoes no pH changes or effect on vaginal flora.

Contrelle Activgard: Summary Individual trials
Viveca Biomed are pleased to confirm they will shortly be commencing a new multi centre clinical trial in the UK which is expected to be the largest trial in bladder leakage in Europe in recent years.
Medical Device Class IIa
Contrelle Activgard is a medical device which conforms with the Essential Requirements of the Medical Device Directive - 93/42/EEC of June 14, 1993 as amended by Directive 2007/47/EC of 21st September 2007. It is designed and manufactured by Viveca Biomed Limited, in accordance with the scope of a quality system which meets the requirements of ISO 13485:2016 and the Medical Devices Directive - 93/42/EEC.
The Notified Body is ( No. 1282) ENTE Certificazione Macchine SRL (ECM), Via Ca’ Bella, 243/A – loc. Castello di Serravalle 40053 Valsamoggia (BO) Italy who have approved the manufacturing full quality assurance system.
CS Life Sciences Europe Ltd, 3 Inns Quay, Dublin 7, Ireland has been appointed to act for Viveca Biomed Ltd within the European Union as their Authorised Representative.
GMDN code 61853
EUDAMED Registration No. SRN GB-MF 000012584





